- Open the white cap
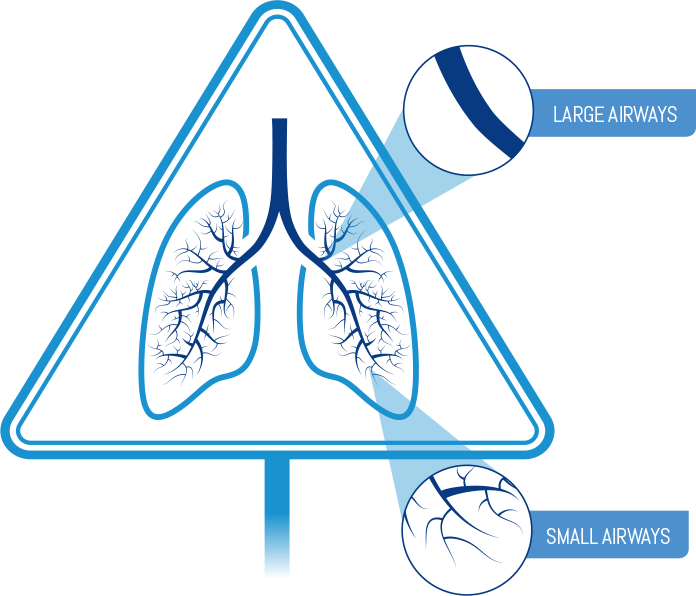
- Breathe out fully
Remember: Do not open the cap until you are ready to take your inhalation and never breathe out into the inhaler mouthpiece.
ICS=inhaled corticosteroid.
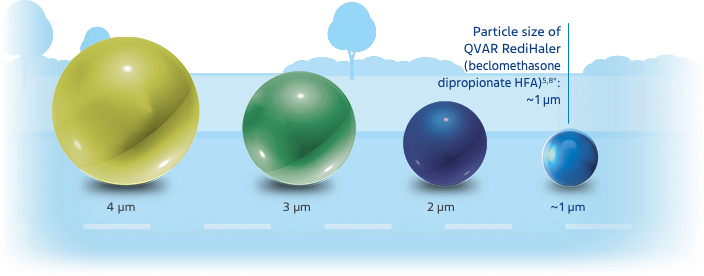
The relationship of particle size to clinical efficacy is unknown.
HFA=hydrofluoroalkane.
Chart not drawn to scale.
*Particle size measures are mass median aerodynamic diameter (MMAD).
The relationship of lung deposition to clinical efficacy is unknown. While deposition studies have not been conducted with QVAR RediHaler, data from QVAR, which is bioequivalent to QVAR RediHaler, show that the majority of medication is deposited in the lungs, not the oropharynx.3,10
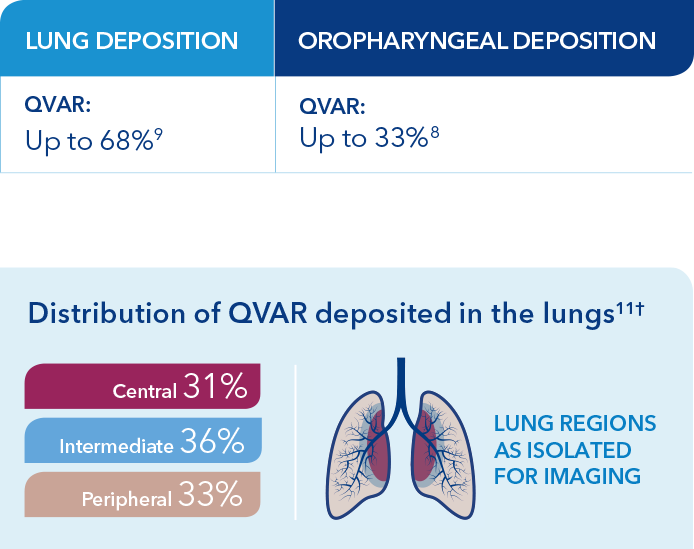
†Based on an open-label study in 7 patients with mild asthma designed to determine the lung deposition and distribution of radiolabeled HFA-BDP from 2 devices, using coordination and discoordinated techniques in the press and breathe (P&B) inhaler. The distribution shown here is for the P&B on-time group, who were trained to actuate 0.32 seconds after start of inspiration.11
‡<60 L/min is considered to be a low inspiratory flow rate.


Hand–breath coordination with pMDIs can be a struggle

Spacers are not always a solution
§Do not use with a spacer.
pMDI=pressurized metered-dose inhaler.
Read complete Instructions for Use in full Prescribing Information
Remember: Do not open the cap until you are ready to take your inhalation and never breathe out into the inhaler mouthpiece.
Remember: Hold inhaler upright as you take your inhalation and do not cover the vents on top.
Remember: If your healthcare provider has told you to take more than 1 inhalation per dose, make sure the white cap is closed and repeat steps 1-3. After taking your prescribed number of inhalations, rinse your mouth with water without swallowing to help reduce the risk of a fungal infection (thrush) in your mouth.
IMPORTANT: Always close the cap after use. This primes the device for the next dose.
To trigger asthma medication release, breath-actuated devices are dependent on inspiratory flow, which varies among patients20,21
The average inspiratory flow rate for tidal breathing (breathing at rest) of young children is 8 to 16 L/min23
The average inspiratory flow rate for tidal breathing of adults is 13 to 18 L/min23
Inspiratory flow rate required to actuate QVAR RediHaler
The propellant hydrofluoroalkane in QVAR RediHaler assists in dose delivery by propelling medication out of the device after a patient takes an inhalation12
*<60 L/min is considered to be a low inspiratory flow rate.
References: 1. Gelfand EW, Kraft M. The importance and features of the distal airways in children and adults. J Allergy Clin Immunol. 2009;124(6 Suppl):S84-S87. 2. Martin RJ. Therapeutic significance of distal airway inflammation in asthma. J Allergy Clin Immunol. 2002;109(2 Suppl): S447-S460. 3. Leach CL, Kuehl PJ, Chand R, Ketai L, Norenberg JP, McDonald JD. Characterization of respiratory deposition of fluticasone-salmeterol hydrofluoroalkane-134a and hydrofluoroalkane-134a beclomethasone in asthmatic patients. Ann Allergy Asthma Immunol. 2012;108(3):195-200. 4. Zeidler M, Corren J. Hydrofluoroalkane formulations of inhaled corticosteroids for the treatment of asthma. Treat Respir Med. 2004;3(1):35-44. 5. Data on file (R&D Investigation Report: Assessment of Aerodynamic Particle Size Distribution of BDP BAI and QVAR MDI). Parsippany, NJ: Teva Respiratory, LLC. November 2017. 6. van der Molen T, Postma DS, Martin RJ, et al. Effectiveness of initiating extrafine-particle versus fine-particle inhaled corticosteroids as asthma therapy in the Netherlands. BMC Pulm Med. 2016;16(80):1-10. 7. National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma–Full Report 2007. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; August 2007. 8. Leach CL, Davidson PJ, Boudreau RJ. Improved airway targeting with the CFC-free HFA–beclomethasone metered-dose inhaler compared with CFC–beclomethasone. Eur Respir J. 1998;12(6):1346-1353. 9. Dolovich M, Labiris R. Imaging drug delivery and drug responses in the lung. Proc Am Thorac Soc. 2004;1:329-337. 10. Small CJ, Gillespie M. Pharmacokinetics of beclomethasone dipropionate delivered by breath-actuated inhaler and metered-dose inhaler in healthy subjects. J Aerosol Med Pulm Drug Deliv. 2018;31(3):182-190. 11. Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA-beclomethasone from a metered dose inhaler. J Aerosol Med. 2005;18(4):379-385. 12. QVAR RediHaler Prescribing Information. Parsippany, NJ: Teva Respiratory, LLC. 13. Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw Q, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224. 14. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19(2):246-251. 15. Khassawneh BY, Al-Ali MK, Alzoubi KH, et al. Handling of inhaler devices in actual pulmonary practice: metered-dose inhaler versus dry powder inhalers. Respir Care. 2008;53(3):324-328. 16. Wilkes W, Fink J, Dhand R. Selecting an accessory device with a metered-dose inhaler: variable influence of accessory devices on fine particle dose, throat deposition, and drug delivery with asynchronous actuation from a metered-dose inhaler. J Aerosol Med. 2001;14(3):351-360. 17. Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50(10):1360-1374. 18. Guilbert TW, Colice G, Grigg J, et al. Real-life outcomes for patients with asthma prescribed spacers for use with either extrafine- or fine-particle inhaled corticosteroids. J Allergy Clin Immunol Pract. 2017;5(4):1040-1049. 19. Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36(2):213-218. 20. Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13. doi:10.1186/s40248-015-0012-5. 21. Lavorini F. The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy. 2013;102418. doi:10.1155/2013/102418. 22. Data on file (Breath actuated devices and inspiratory flow rates). Parsippany, NJ: Teva Respiratory, LLC. April 2019. 23. Haidl P, Heindl S, Siemon K, Bernacka M, Cloes RM. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75.